Research
Dr. Devin O’Brien Coon’s lab is a basic science lab studying the biology of skin, soft tissue and the extracellular matrix. Specific areas of research inquiry include regenerative medicine, organ tissue engineering and scarring/fibrosis within post-surgical and pathologic settings. Check out our active areas of research in the tabs below!
Fibrosis is a pathological accumulation of excessive collagen that underlies many of the most common diseases. This process represents the dysfunction of normal tissue healing. Fibrosis research aims to limit this response without effecting the essential role of fibrogenesis in organ function and recovery from injury. However, the absence of a realistic in vitro model has hindered investigation into mechanisms and potential interventions: the standard 2D monolayer culture of fibroblasts used up to this point has limited applicability.
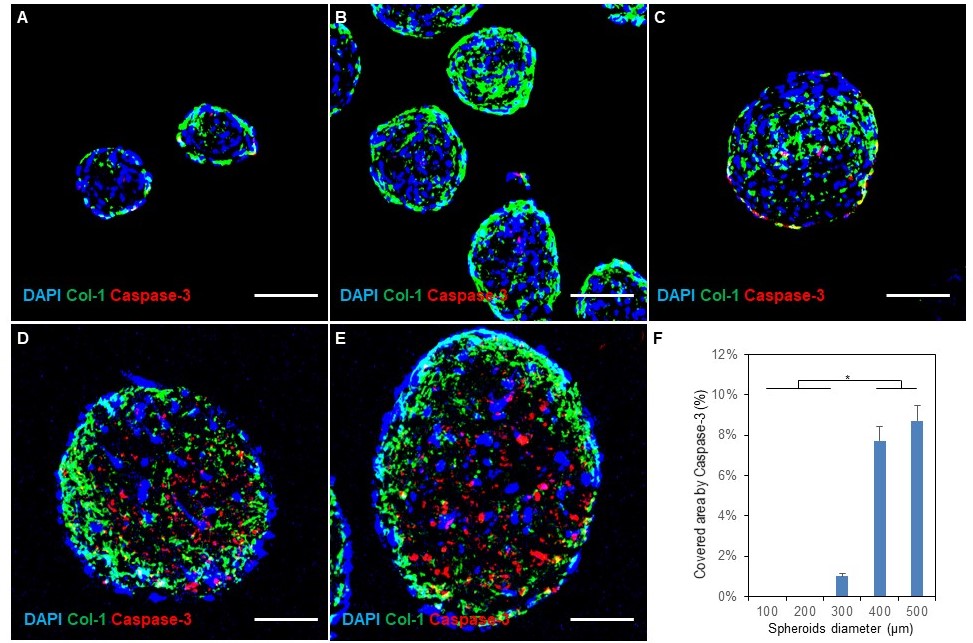
In the Coon lab, we have developed and optimize fibrosis spheroids: a scaffold-free three-dimensional human fibroblast-macrophage spheroid system representing an improved in vitro model of human fibrosis. We created, characterized and optimized human fibroblast-only spheroids, demonstrating increased collagen deposition compared to monolayer fibroblasts. Next, we improved the spheroid system with the addition of human macrophages to more precisely repeat the environment during fibrogenesis, creating a hybrid spheroid system with different ratios of fibroblasts and macrophages ranging from 2:1 to 64:1. We found that in the hybrid spheroids more fibroblasts were activated, with greater macrophage polarization towards a pro-inflammatory M1 phenotype. Hybrid spheroids containing higher ratios of macrophages showed greater macrophage heterogeneity and less fibrogenesis, while low macrophage ratios limited macrophage-induced effects and yielded less collagen deposition.
Future studies exploring the greater fibrotic activity of these spheroids may provide new mechanistic insights into diseases involving excessive fibrotic activity. Microtissue fibrosis models capable of achieving greater clinical fidelity have the potential to combine the relevance of animal models with the scale, cost and throughput of in vitro testing.
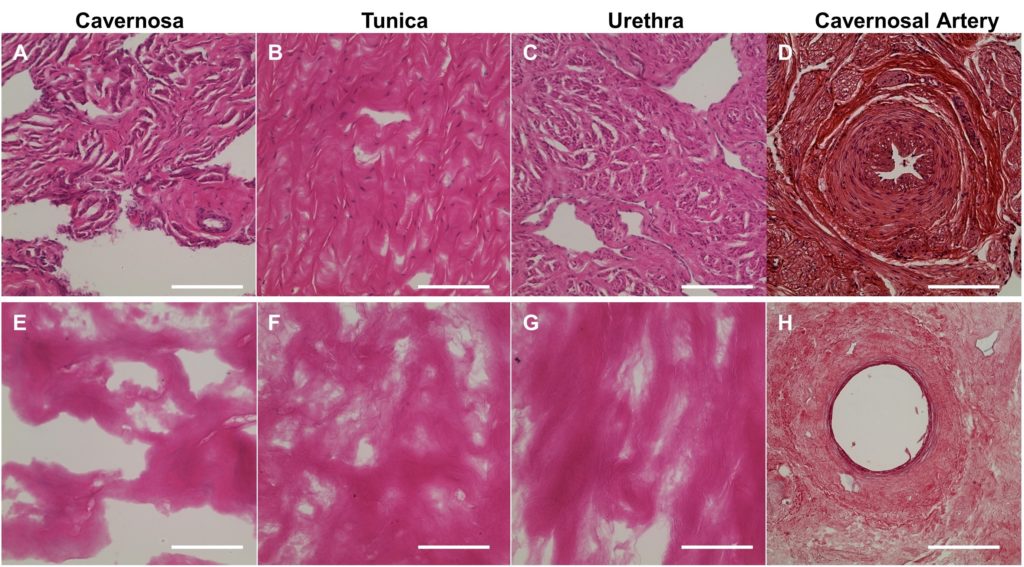
Reconstruction for total penile defects presents unique challenges due to the organ’s complexity. Current standards in care include high rates of complications and relatively low longterm success rates. In our lab, we have developed the first comprehensive protocol for decellularizing whole-organ human penile specimens, for use in total penile tissue engineering.
The use of a hybrid decellularization scheme combining micro-arterial perfusion, urethral catheter perfusion and external diffusion enabled the creation of a full-size scaffold with removal of immunogenic components. Decellularization was completed and evaluated using a variety of methods. An intact ECM was maintained with histologic architecture. Post-decellularization patency of the cavernosal arteries for future use in reseeding was demonstrated. Scaffold biocompatibility was evaluated using human adipose-derived stromal vascular cells. Influence on cellular behavior was seen with significantly higher expression of VWF, COL1, SM22 and Desmin as compared to cell monolayer. Preliminary evidence for regional tropism was also seen, with formation of microtubules and increased endothelial marker expression in the cavernosa.
This report of successful decellularization of the complete human phallus is an initial step towards developing a tissue engineered human penile scaffold with potential for more successfully restoring cosmetic, urinary and sexual function after complete penile loss.